Regulatory
Symbol glossary
 | CE mark |
 | Korean Certification (KC) Mark |
 | Australia Radiocommunications and EMC Compliance Mark |
 | Waste Electrical and Electronic Equipment symbol |
 | Manufacturer |
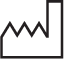 | Date of manufacture |
 | Reference Number |
 | Serial Number |
 | Li-ion batteries recycling symbol |
USA Federal Communications Commission (FCC) Statement
This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions:
This device may not cause harmful interference.
This device must accept any interference received, including interference that may cause undesired operation.
FCCID: QHQ-212-00001(Drug Arm only)
Caution: Changes or modifications not expressly approved by Laerdal Medical could void the user's authority to operate the equipment.
For SimMan 3G, SimMan 3G PLUS, SimMan Essential, SimMan Essential Bleeding and SimMan Trauma:
This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to part 15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference in a residential installation. This equipment generates, uses and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to radio communications. However, there is no guarantee that interference will not occur in a particular installation.
If this equipment does cause harmful interference to radio or television reception, which can be determined by turning the equipment off and on, the user is encouraged to try to correct the interference by one or more of the following measures:
Reorient or relocate the receiving antenna.
Increase the separation between the equipment and receiver.
Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.
Consult the dealer or an experienced radio/TV technician for help.
EU
CE statement
The product is in compliance with Council Directive 2011/65/EU on restriction of the use of certain hazardous substances (RoHS).
For SimMan manikins with the articulated arm:
The product is in compliance with the essential requirements of Council Directive 2014/30/EU on electromagnetic compatibility (EMC), and the product is in compliance with cyber security requirements of Radio Equipment Direction (RED) Article 3.3.
For SimMan manikins with drug arm (RFID):
This product is in compliance with the essential requirements of Council Directive 2014/53/EU on Radio Equipment (RED).
Canada
Canadian ICES-003 Statement
This Class B digital apparatus complies with Canadian ICES-003(B).
Cet appareil numérique de la classe B est conforme à la norme NMB-003(B) du Canada.
Korean Certification
MSIP-CMI-LMQ-SM3G (SimMan 3G/Essential/Essential Bleeding/Trauma)